IMPORTANT FDA UPDATE REGARDING IV FLUIDS SITUATION: Extension of the Expiration Date for 10% LMD in 0.9% Sodium Chloride Injection
 This is to provide you with important information regarding 10% LMD in 0.9% Sodium Chloride Injection (Dextran 40 in Sodium Chloride Injection, USP) and 10% LMD in 5% Dextrose Injection (Dextran 40 in Dextrose Injection, USP) manufactured by Hospira Inc., a Pfizer company.
This is to provide you with important information regarding 10% LMD in 0.9% Sodium Chloride Injection (Dextran 40 in Sodium Chloride Injection, USP) and 10% LMD in 5% Dextrose Injection (Dextran 40 in Dextrose Injection, USP) manufactured by Hospira Inc., a Pfizer company.
Based upon FDA’s evaluation of stability data, FDA has extended the expiration date on one lot of 10% LMD in 0.9% Sodium Chloride Injection (lot # 5859955) and one lot of 10% LMD in 5% Dextrose Injection (lot # 6040410) as follows:
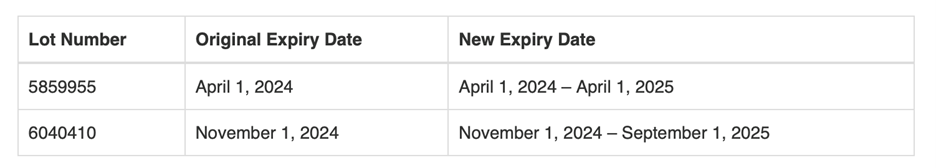
The FDA is not requiring or recommending that the lots identified above be relabeled with new expiration dates. If you have bags of lot #5859955 in your current inventory, labeled with an expiration date of April 1, 2024, you may continue to maintain the product in your inventory and keep it available for use until April 1, 2025. If you have bags of lot #6040410 in your current inventory, labeled with an expiration date of November 1, 2024, you may continue to maintain the product in your inventory and keep it available for use until September 1, 2025.
Please use available inventory on a first-in, first-out basis through the new expiration dates as noted above.
We encourage all AHVAP members to visit our Emergency Supply Chain Disruption Resource Center for the most up-to-date information. In addition, AHVAP's webinar from earlier today is now available on this website as well.
To view the full FDA communication, visit the FDA's posting here.