Authors
Christopher M. Leibundguth
David C. Lyons
Bradley A. McKinney
Other Authors
Steven B. Toeniskoetter
On Thursday, June 26, 2014, the U.S. Food and Drug Administration (FDA) held a public meeting in Washington, D.C. to discuss the two proposed rule changes to the Nutrition Facts panels seen on most packaged foods in the U.S. These would be the first substantive changes made to the labels since 2003.
Overview of Proposed Rules
The first proposed rule, Food Labeling: Revision of the Nutrition and Supplement Facts Labels, contains proposed changes to the format of the label, as well as proposed changes to which nutrients should be required to be listed on the label. Specifically, suggested changes include:
- Increasing the prominence of the calories and serving size listing
- Removing "Calories from Fat" from the label
- Requiring information about the amount of "added sugars" in a food product
Below are illustrations of the current and proposed labels.
|
Current
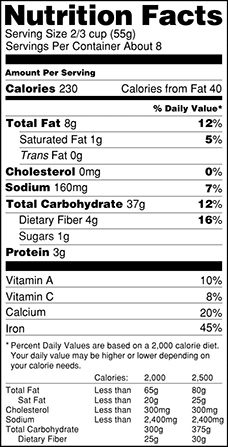 |
Proposed
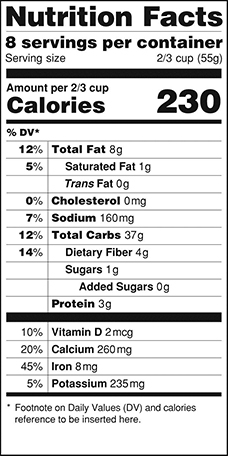 |
The second proposed rule, Food Labeling: Serving Sizes of Foods That Can Reasonably Be Consumed At One-Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments, would alter serving sizes to better reflect portions actually consumed in one setting, also referred to as "reference amounts customarily consumed" (RACCs). For instance, a 20 ounce beverage's label would read "Amount Per Bottle" rather than "Amount Per Serving."
The following FDA staff members, each of whom played a role in drafting the proposed rules, were on hand to engage with stakeholders:
- Michael Taylor, Deputy Commissioner for Foods and Veterinary Medicine
- Michael Landa, Director, FDA Center for Food Safety and Applied Nutrition (CFSAN)
- Paula Trumbo, Team Leader, Nutrition Science Review, Nutrition Programs, CFSAN
- Jillonne Kevala, Team Leader, Nutrition Assessment and Evaluation, Nutrition Programs, CFSAN
- Sharon Natanblut, Senior Advisor for Strategic Communications and Public Engagement
Takeaways from the Public Meeting
Representatives from FaegreBD Consulting covered the public meeting and noted the following key takeaways:
- Almost all of stakeholders expressed the importance of FDA's proposed changes to the Nutrition Facts panel, often specifically citing the proposed changes to serving sizes.
- Stakeholder commentary included concerns with the requirement for listing "added sugars," changes to the serving size format, the decrease in "daily value" for sodium, and the timeline for implementation if and when a final rule is issued.
- There was dispute from various stakeholders regarding "added sugars," with some questioning the scientific basis of the provision, while others spoke in support of an "added sugars" label, citing concerns about obesity.
- With regard to implementation timeline, some stakeholders suggested the proposed two-year window is insufficient and requested an extension to grant businesses three to five years.
- Other topics included the notion of adding phosphorus to labels to aid those with kidney disease, and potential consumer confusion over including "added fiber" that is not associated with normal dietary fiber.
The rules were initially published in the Federal Register on March 3, 2014. The comment period, originally set to end June 2, 2014, was extended to August 1, 2014.